BioPur Process
Move to a regenerative, healing, and care model:
Unlike small molecules, interventions derived from human tissue up-regulate the body’s ability to self-heal.
Allopathic treatments address a wide spectrum of medical and surgical pathologies, without side effects and with successful patient outcomes.
New Customer Inquiry
Place an Order For
BioXtek’s Placental membrane solution:
Preserving the ECM components providing:
A scaffold to support native tissue when traumatized.

Sanoplast UAP
Human Umbilical cord powder
• Lyophilized umbilical cord, micronized.
• Stored at ambient temperatures.
Versatile Applications:
Designed for a wide range of medical uses, including wound care, burns and wounds.
Offering unmatched versatility and effectiveness.
Our advanced proprietary BioPur Process ensures the highest quality and safety by preserving key bioactive molecules, removing impurities, and eliminating the risk of potential adverse reactions.
CordGuardECM Human Umbilical cord patches
- Single layer umbilical cord allograft
- Lyophilized and cut into patches
- Preserving the ECM components providing : A scaffold to support native tissue when traumatized.
- Can be sutured.
- Stored at ambient temperatures.
Umbilical membrane allografts are derived from a prescreened mother with a healthy non emergent delivery. Manufactured in
compliance with FDA and AATB regulations & guidance. The membrane tissue is minimally manipulated to preserve the native structure of the tissue and terminally sterilized. Confirmed by the FDA tissue reference group to meet criteria for regulation solely under Section 361 PHS 21CFR Part 1271

White labeling available
General Information:
Ordering Information
| Product Name | Size | Product Number |
|---|---|---|
| Sanoplast UAP – human umbilical cord powder | 1ml in 2ml vial | UAP-0010 |
| CordGuardECM – human umbilical cord patch 1x2 | 1x2cm | UCP-0102 |
| CordGuardECM – human umbilical cord patch 2x2 | 2x2cm | UCP-0202 |
| CordGuardECM – human umbilical cord patch 2x3 | 2x3cm | UCP-0203 |
| CordGuardECM – human umbilical cord patch 2x4 | 2x4cm | UCP-0204 |
| CordGuardECM – human umbilical cord patch 3x5 | 3x5cm | UCP-0305 |
SANOPLAST ECM
Amniotic Membrane Allograft
Sanoplast ECM Amniotic Membrane is a sterile single amnion layer allograft designed for optimal wound covering and protection during the treatment of wounds.
Features & Properties
- Provides a protective wound covering
- Dehydrated extracellular matrixs acts as a scaffold supporting the native issue
- Adheres easily to wounds including those with irregular surfaces
- Ambient temperature storage
Amniotic membrane allografts are derived from a prescreened
mother with a healthy non emergent delivery. Manufactured in
compliance with FDA and AATB regulations & guidance. The
membrane tissue is minimally manipulated to preserve the native
structure of the tissue and terminally sterilized. Confirmed by the
FDA tissue reference group to meet criteria for regulation solely
under Section 361 PHS 21CFR Part 1271

SanoOptic
10mm eye patches single layer amnion OPT-0010
White labeling available
General Information:
Reimbursement and coverage eligibility for the use of Single Layer Sanoplast ECM Membrane and associated Procedures varies by Medicare and private payers. Coverage policies, prior authorizations, contract terms, billing edits and site of service influence reimbursement.
Ordering Information
| Product Number | Size | Total Units(Per SQCM) |
|---|---|---|
| SCM-0202 | 2x2 | 4 |
| SCM-0203 | 2x3 | 6 |
| SCM-0404 | 4x4 | 16 |
| SCM-0406 | 4x6 | 24 |
| SCM-0408 | 4x8 | 32 |
| SCM-1020 | 10x20 | 200 |
| SCM-1520 | 15x20 | 300 |

SANOPLAST DUO
Amniotic Membrane Allograft
Sanoplast Duo Amniotic membrane is a sterile dual layer allograft designed for optimal wound covering and protection during the treatment of wounds.
Features & Properties
- Provides a protective wound covering
- Dehydrated extracellular matrixs acts as a scaffold supporting the native issue
- Adheres easily to wounds including those with irregular surfaces
- Ambient temperature storage
- Tri and Quad layer versions available
- Tri layer is amnion chorion amnion
- Quad layer is amnion chorion chorion amnion
Amniotic membrane allografts are derived from a prescreened mother with a healthy non emergent delivery. Manufactured in compliance with FDA and AATB regulations & guidance. The membrane tissue is minimally manipulated to preserve the native structure of the tissue and terminally sterilized. Confirmed by the FDA tissue reference group to meet criteria for regulation solely under Section 361 PHS 21CFR Part 1271
White labeling available
General Information
Reimbursement and coverage eligibility for the use of Dual Layer Sanoplast Duo Membrane and associated procedures varies by Medicare and private payers. Coverage policies, prior authorizations, contract terms, billing edits, and site-of- service influence reimbursement.
Sanoplast Duo Ordering Information
| Product Number | Size | Total Units(Per SQCM) |
|---|---|---|
| SAA-0202 | 2x2 | 4 |
| SAA-0203 | 2x3 | 6 |
| SAA-0404 | 4x4 | 16 |
| SAA-0406 | 4x6 | 24 |
| SAA-0408 | 4x8 | 32 |
| SAA-1020 | 10x20 | 200 |
| SAA-1520 | 15x20 | 300 |
Membrane patches
Therapeutic applications
7 existing products are commercialized and sold direct and via distributors for these applications:
- Dry eye syndrome
- Diabetic wounds
- 2nd /3rd degree burns
- Cardiac pericardial replacement
- Dura patch for spine and neuro surgery
- Intra operative anti scar patch
Competitive advantage
Utilizes BioPur process to ensure cell free stable extracellular matrix to provide a consistent membrane patch.
Clinical results demonstrating significant consistent wound healing
Versatile Applications: Designed for a wide range of medical uses, including wound care, burn treatment, dura replacement, and tendon and nerve repair, offering unmatched versatility and effectiveness
Proprietary BioPur Process: Our advanced BioPur Process ensures the highest quality and safety by preserving key bioactive molecules, removing impurities, and eliminating the risk of immune reactions.
Regulatory pathway
Transition our placental membrane patches from FDA 361 status to FDA 510k medical device clearance.
Commercialization
Beyond our existing wound-care products will unlock broader insurance reimbursement and expand our market reach beyond wound care into surgical specialties such as neurosurgery, gynecology, dermatology, cardiothoracic surgery, and orthopedics.
Case study 1
40-year old female, 5 years post double mastectomy, non-healing radiation burn multiple failed skin grafts and surgeries to repair.
1st treatment with Sanoplast membrane. Within 3 weeks, the wound is healed!
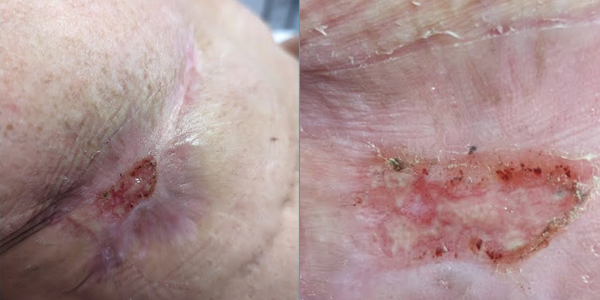
Adult skin regenerated like young tissue in response to the proteins found in Purified Amniotic Fluid (hPAF) exosomes.
Reduction of pain within 30 minutes; patient able to stop opioids for pain management.
Doctors expected 2-3 month recovery and were amazed at the recovery that would have required extensive skin grafting.
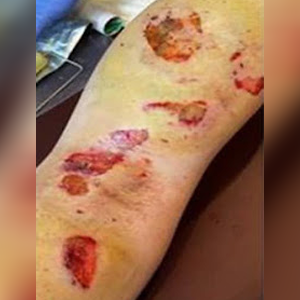
Day 1 of treatment
Third-degree burns day 2 post injury
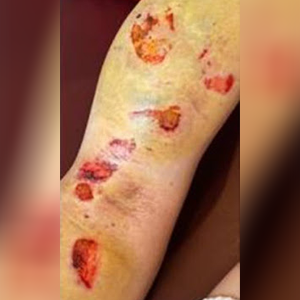
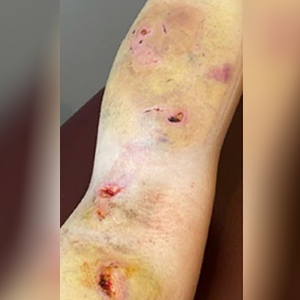
10 days post-topical treatment

Mother Nature’s Miracle
Mother Nature's Miracle- the optimal natural elixir for growth and development.
A topical cosmetic with unadulterated EXOSOMES derived from pure amniotic fluid suspension and the nourishing layers of the umbilical cord (aka Wharton’s Jelly), to preserve Mother Nature’s perfection in creating the optimal environment for potential facial skin improvement.
- May make fine lines and wrinkles less noticeable.
- May reduce the signs of aging by moisturizing dry skin.
- May improve the skin tone and elasticity
- Can improve discolorations of the skin
Revolutionizing Skincare
The product contains Exosomes derived from human Amniotic Fluid, and Wharton’s Jelly.
Future Therapeutic
Injectable regenerative therapeutics from contents of umbilical cord and amniotic fluid.
Therapeutic: Fluid Implantable Allograft
Future Potential Therapeutic applications - when approved by US FDA
- Wound care
- Cardiopulmonary
- Clinic: orthopedic, dermatology, pain mitigation
- Dental
- Surgical: orthopedic, neuro/spine, plastic and general general
- Aesthetics
Regulatory pathway
Heading towards IND application submission having completed validated process formula, full quality system implementation. Completing the full Characterization of the actual therapeutic presently.
Passed audit of the American Association of Tissue Banks
Currently have 2 IRB approved clinical trials
Competitive advantage
Averaging 350B protein particles carrying a varying exosome cargo of miRNA, MRNA, proteins, lipids, Growth Factors, Peptides, ECM/collagens, Hyaluronic Acid.
Minimally manipulated concentrate. Will be available in several forms cryopreserved at -80c
or
Freeze-dried
Commercialization
Establish Distribution Channels
Marketing and Branding
Sales Strategy
Reimbursement and Payer Strategy
Clinical Support and KOL Engagement
International Expansion
Continuous Improvement and Innovation
Cosmetic products
Our premium topical aesthetic product is designed to maximize the effects of topically applied amniotic fluid and Wharton’s jelly.
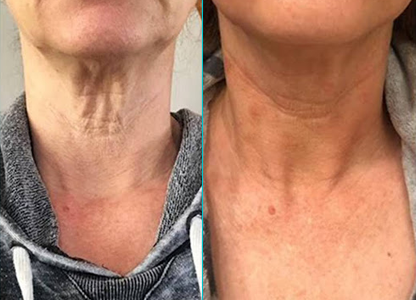
Aesthetic applications
- Facial aesthetics
- Hair aesthetics
- Topical Scar application
- Collagen Stimulation Hydration and Nourishment
Competitive advantage
These products support the delivery of the entire ‘symphonic orchestra’ needed for regenerative aesthetics.
This product theoretically bolsters the building of robust collagen.
Regulatory pathway
US FDA Registered Topical Cosmetic
Full compliance with US FDA cosmetic regulations
Commercialization
Product currently sold directly and via distribution channels to longevity clinics, medi-spas, and licensed professionals.

